Abstract
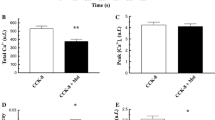
Hyperstimulation with cholecystokinin analogue cerulein induces a mild edematous pancreatitis in rats. There is evidence for a diminished energy metabolism of acinar cells in this experimental model. The aim of this study was to demonstrate permeability transition of the mitochondrial inner membrane as an early change in mitochondrial function and morphology. As functional parameters, the respiration and membrane potential of mitochondria isolated from control and cerulein-treated animals were measured, and changes in volume and morphology were investigated by swelling experiments and electron microscopy. Five hours after the first injection of cerulein, the leak respiration was nearly doubled and the resting membrane potential was decreased by about 17 mV. These alterations were reversed by extramitochondrial ADP or did not occur when cyclosporin A was added to the mitochondrial incubation. A considerable portion of the mitochondria isolated from cerulein-treated animals was swollen and showed dramatic changes in morphology such as a wrinkled outer membrane and the loss of a distinct cristae structure. These data provide evidence for the opening of the mitochondrial permeability transition pore at an early stage of cerulein induced pancreatitis. This suggests that the permeability transition is an initiating event for lysis of individual mitochondria and the initiation of apoptosis and/or necrosis, as had been shown to occur in this experimental model.
Similar content being viewed by others
References
Frade JM, Michaelidis TM: Origin of eukaryotic programmed cell death: A consequence of aerobic metabolism? Bioessays 19: 827–832, 1997
Simbula G, Glascott PA Jr, Akita S, Hoek JB, Farber JL: Two mechanisms by which ATP depletion potentiates induction of the mitochondrial permeability transition. Am J Physiol 273: C479–C488, 1997
Zamzami N, Hirsch T, Dallaporta B, Petit PX, Kroemer G: Mitochondrial implication in accidental and programmed cell death: Apoptosis and necrosis. J Bioenerg Biomembr 29: 185–193, 1997
Kuroda S, Siesjo BK: Reperfusion damage following focal ischemia: Pathophysiology and therapeutic windows. Clin Neurosci 4 199–212, 1997
Gunter TE, Pfeiffer DR: Mechanisms by which mitochondria transport calcium. Am J Physiol 258: 755–786, 1990
Bernardi P, Vassanelli S, Veronese P, Calona R, Szabo I, Zoratti M: Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem 267: 2934–2939, 1992
Medina V, Edmonds B, Young GP, James R, Appleton S, Zalewski PD: Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin A (inhibitors of histone deacetylase): Dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Res 57: 3697–3707, 1997
Liu X, Kim CN, Yang J, Jemmerson R, Wang X: Induction of apoptotic program in cell-free extracts: Requirement for DATP and cytochrome c. Cell 86: 147–157, 1996
Kristian T, Siesjo BK: Calcium-related damage in ischemia. Life Sci 59: 357–367, 1996
Aguilar HJ, Botia R, Arora AS, Bronk SF, Gores GJ: Induction of the mitochondrial permeability transition by protease activity in rats: A mechanism of hepatocyte necrosis. Gastroenterology 110: 558–566, 1996
Halangk W, Matthias R, Schild L, Meyer F, Schuiz HU, Lippert H: Effect of supramaximal cerulein stimulation on mitochondrial energy metabolism in rat pancreas. Pancreas 16: 88–95, 1998
Ward JB, Petersen OH, Jenkins SA, Sutton R: Is an elevated concentration of acinar cytosolic free calcium the trigger for acute pancreatitis? Lancet 346: 1016–1019, 1995
Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML: Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol 269: C1295–C1304, 1995
Halestrap AP, Connern CP, Griffith EJ, Kerr PM: Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischemia/reperfusion injury. Mol Cell Biochem 174: 167–172, 1997
Lemasters JJ, Nieminen AL, Oian T, Trost LC, Herman B: The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol Cell Biochem 174: 159–165, 1997
Wilson JS, Korsten MA, Leo MA, Lieber CS: New technique for the isolation of functional rat pancreatic mitochondria and its application to models of pancreatic injury. J Lab Clin Med 107: 51–58, 1986
Reynafarje B, Costa LE, Lehninger AL: O2 sobility in aqueous media determined by a kinetic method. Anal Biochem 145: 406–418, 1985
Kamo N, Muratsugu M, Hongoh R, Kobatake Y: Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol 49: 105–121, 1979
Schmidt E: Methoden der enzymatischen Analyse. In: HU Bergmeyer (ed). Akademie-Verlag, Berlin, 1970, 607–613
Massari S: Kinetic analysis of the mitochondrial permeability transition. J Biol Chem 271: 31942–31948, 1996
Wolf G, Wurdig, S, Henschke G: Nitric oxide synthase in the brain: Light and electron microscopical findings based on the NADPH-diaphorase reaction. J Neural Trans Suppl 43: 105–112, 1994
Gorelick FS, Adler G, Kern HF: Cerulein induced pancreatitis. In: VLW Go, EP Di Magno, JD Gardner, E Lebenthal, HA Reber, GA Scheele (eds). The Pancreas: Biology, Pathology, and Disease. Raven Press, New York, 1993, pp 501–526
Lüthen R, Niederau C, Grendell JH: Interpancreatic zymogen activation and levels of ATP and glutathione during caerulein pancreatitis in rat. Am J Phys 268: G592–604, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schild, L., Stanarius, A., Wolf, G. et al. Induction of permeability transition in pancreatic mitochondria by cerulein in rats. Mol Cell Biochem 195, 191–197 (1999). https://doi.org/10.1023/A:1006988625831
Issue Date:
DOI: https://doi.org/10.1023/A:1006988625831