Article Text
Abstract
Objectives To assess the incidence of biopsy-verified coeliac disease (CD) in Sweden and examine the incidence of duodenal/jejunal biopsies with normal mucosa over time as a proxy for CD awareness and investigation.
Design Nationwide population-based cohort study 1990–2015 based on biopsy reports indicating villous atrophy (VA) or normal mucosa in the duodenum/jejunum.
Results We identified 44 771 individuals (63% females) with a biopsy report specifying VA and 412 279 (62% females) with a biopsy report indicating normal mucosa (without a prior biopsy indicating VA). The median age at diagnosis of CD was 28 years. The mean age-standardised incidence rate during the study period was 19.0 per 100 000 person-years (95% CI 17.3 to 20.8). The incidence reached a peak in 1994 for both sexes and a second higher peak in 2002–2003 for females and in 2006 for males. The lifetime risk of developing CD was 1.8% (2.3% in females and 1.4% in males).
Prior to 2015, there was a parallel rise in rates for biopsies showing normal duodenal/jejunal mucosa.
Conclusions In Sweden, the incidence of CD increased until 2002–2003 in females and until 2006 in males. Since then, the incidence of CD has declined despite increasing duodenal/jejunal biopsies, suggesting that increased awareness and investigation are unlikely to elevate the incidence of the disease in Sweden. Across a lifetime, 1 in 44 females and 1 in 72 males are expected to be diagnosed with CD in Sweden, indicating a relatively high societal burden of disease.
- coeliac disease
- epidemiology
Data availability statement
In accordance with Swedish regulation the data from this study are not publicly available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Significance of this study
What is already known on this subject?
Coeliac disease (CD) is a common condition linked to increased morbidity and mortality. Figures on incidence, however, have varied. Because of specific diagnostic procedures and sometimes mild symptoms, awareness and diagnostic resources may affect disease incidence.
What are the new findings?
Thanks to this study’s nationwide coverage, we can present accurate data without the risk of selection bias. Moreover, data on normal biopsies provided context for our findings as they reflect awareness and investigation of CD. The large study population allowed us to calculate a precise estimate of the lifetime risk of being diagnosed with CD.
How might it impact on clinical practice in the foreseeable future?
Our results suggest that increased awareness and level of investigation for CD in Sweden are unlikely to impact incidence rates. This knowledge may assist in deciding on allocation of resources in the future.
Introduction
Coeliac disease (CD) is an immune-mediated disorder characterised by small intestinal inflammation and villous atrophy (VA).1 CD constitutes a substantial societal burden of disease owing to its high prevalence (global prevalence of biopsy-verified CD has been estimated at 0.7%2), its chronic course, the association with increased mortality3 and morbidity4 (including lymphoma5), restrictions in diet6 7 and psychosocial stress associated with lower quality of life.8
While there is an abundance of studies on the prevalence of CD,2 fewer large-scale studies are available on disease incidence (online supplemental table S1). Temporal shifts in incidence are important because they are unlikely to be caused by genetic factors alone9 but can instead highlight environmental risk factors, the impact of detection strategies and avenues for intervention in the long term.
Supplemental material
Throughout the latter half of the 20th century and into the 21st, Northern countries—particularly Sweden, Finland and Norway—have seen some of the highest incidence rates of CD.10–12 However, a recent systematic review has shown a consistent trend of rising incidence throughout many industrialised countries over the past several decades,13 underlining an increased prominence of CD on a global scale. While incidence itself has historically been higher in children, diagnosis rates in adults are increasing. Of note, some regions, such as Olmsted County (USA),14 Canterbury (New Zealand)15 and the UK,16 have observed adult-specific rates to be the same or higher than rates in children.17 Moreover, the average age at diagnosis within paediatric populations is increasing in many countries.10 18 19 Therefore, when estimating the incidence of CD and exploring its trends over time in all ages it is crucial to consider these demographic factors.
We retrieved data from all histopathology reports in Sweden through the ESPRESSO study (Epidemiology Strengthened by histoPathology Reports in Sweden).20 Next, we calculated the incidence of CD (defined as equal to VA in the duodenum or jejunum) and the incidence of first biopsy with normal duodenal/jejunal mucosa as a proxy for CD awareness and investigation.
Materials and methods
Setting
We contacted Sweden’s 28 pathology departments to request that information technology (IT) personnel retrieve data on all GI biopsies. Data were organised using the personal identity number, a unique number assigned to all Swedish residents.21 Healthcare in Sweden is universal, tax-funded and based on the principle of need.
CD and normal mucosa
In Sweden, the Systematised Nomenclature of Medicine (SNOMED) system classifies GI biopsies. We asked IT personnel at all pathology departments in Sweden to retrieve data on duodenal/jejunal biopsies exhibiting VA or normal mucosa. These were identified by codes either indicating CD (M58 with subgroups, or the CD diagnostic code D6218) or normal mucosa (SNOMED code M00100 or M00110). The latter group did not include individuals with normal villi but mucosal inflammation. Data collection took place between 12 October 2015 and 10 April 2017. Codes used to identify CD have been used in an earlier study22 encompassing 29 000 individuals with VA (all included in the current study). In that study, and in a later validation study,23 we found that the average small intestinal biopsy report was based on three tissue specimens, that 95% of patients with VA had a clinical diagnosis of CD, and that 172/180 (96%) of Swedish gastroenterologists and 68/68 (100%) paediatricians at the time performed a small intestinal biopsy in at least 9/10 individuals before diagnosis of CD. Some 79% of patients with VA had GI symptoms before biopsy and 88% of individuals with available data on coeliac-related antibodies were positive. To complement the CD validation from 2009,23 one investigator (JFL) manually reviewed the full text of 100 random biopsy reports with VA originating from 2009 to 2017, yielding a positive predictive value (PPV) of 99% (95% CI 94% to 100%).24
For this study, we retrieved data on age, sex and date of diagnosis. The first CD case was diagnosed in 1969, with the first recorded normal mucosa occurring in 1965.
However, because the number of cases before 1990 was relatively low, perhaps indicating an insufficient awareness of CD or limited access to upper endoscopy, we excluded cases from 1969 to 1989. Data collection was considered incomplete for 2016–2017 and therefore excluded from the analysis. We excluded all patients aged ≥100 years (n=15) to minimise misclassification. Finally, we excluded biopsy reports with normal mucosa occurring after a biopsy with CD as such normal biopsies likely reflect mucosal healing.
Patient and public involvement
No patient participated in the planning or design of this study.
Statistics
Incidence rates were age-standardised to the 2015 Swedish population (accessible from Statistics Sweden, www.scb.se) to control for changes in the population’s age structure. To facilitate comparisons, we also calculated age-standardised incidence rates according to the 2013 European standard population structure (https://ec.europa.eu/eurostat/documents/3859598/5926869/KS-RA-13-028-EN.PDF.pdf/e713fa79-1add-44e8-b23d-5e8fa09b3f8f?t=1414782757000) (online supplemental eFigures 1 and 2). We calculated age-standardised incidence rates per 100 000 person-years and age-specific rates by sex. The age-standardised rates were modelled using generalised additive and join-point models using a Gaussian distribution with a log link, weighting by the inverse variance of the rates. Age-specific rates were also modelled using the same models (generalised additive and join-point models) in which the outcomes were assumed to be Poisson distributed, with a log link and a log of person-time included as an offset. We examined whether the incidence rate ratios (IRRs) varied by age and calendar period using generalised additive models.
Supplemental material
Supplemental material
We also estimated the lifetime risk of having an endoscopy with VA or normal appearance by calculating cumulative incidence up to age 85. We based these calculations on the incidence rates of the past 15 years of the study period.
We adopted a p value of <0.05 as a threshold for declaring statistical significance for all statistical analyses. Statistical analyses were performed using Stata V.14.2 (StataCorp) and R V.3.6.3 (R Core Team, Vienna, Austria 2020). The generalised additive models used the mgcv package and the join-point models used the segmented package.25
Ethics
The Regional Ethics Board in Stockholm, Sweden approved the study. Because this was a register-based study, the participants were not contacted.26
Results
Background results
Between 1990 and 2015, we identified 44 771 individuals with an incident diagnosis of CD. The median age at CD diagnosis was 28 years, and 63% were females (table 1).
Patient characteristics
We also identified 412 279 individuals with normal mucosa in the duodenum/jejunum (hereafter referred to as ‘normal mucosa’). Their median age at first normal biopsy was 44 years (62% were females) (table 1).
Calendar period
Coeliac disease
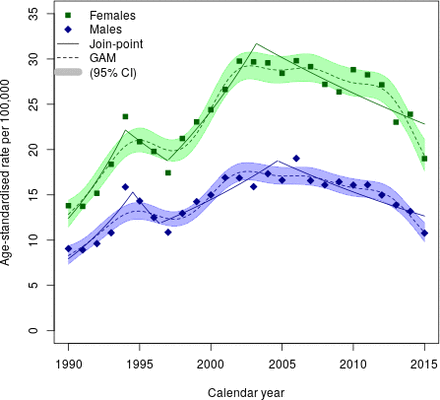
We found two peaks in CD incidence in Sweden during the past 25 years. CD incidence increased until 2002–2003, with a peak incidence rate of 29.7 (95% CI 28.1 to 31.3) per 100 000 person-years for females and until 2006 for males (peak incidence 19.0 per 100 000 person-years, 95% CI 17.7 to 20.3) (figure 1). An earlier peak was observed in 1994 when incidence rates rose to 23.6 (95% CI 22.2 to 25.1) in females and 15.9 (95% CI 14.7 to 17.1) in males. Since the last peak, the overall incidence has decreased slightly despite increasing duodenal/jejunal biopsies.
Age-standardised incidence rate of coeliac disease per 100 000 person-years in Sweden 1990–2015. GAM, generalised additive model.
The mean annual age-standardised incidence rate during the study period was 19.0 cases per 100 000 person-years (95% CI 17.3 to 20.8) (figure 1). Over the past decade of the study period, the mean annual age-standardised incidence rate was 20.8 (95% CI 17.9 to 23.7) per 100 000 person-years. From 1990 to 1999, the mean annual age-standardised incidence rate was 15.3 (95% CI 13.1 to 17.5) per 100 000 person-years. During the last 3 years of the study, incidence declined.
Normal mucosa
During the entire study period, the mean annual age-standardised incidence rate for having a biopsy with normal mucosa was 174.1 per 100 000 person-years (95% CI 154.7 to 193.6) (figure 2). The corresponding figure for 2006–2015 was 220.0 (95% CI 196.5 to 243.5). The incidence increased throughout the study period with the exception of 2015 (figure 2). Stratifying on sex, normal mucosa occurred more frequently in females with a mean incidence rate of 213.8 (95% CI 116.8 to 241.6) throughout the study period. The corresponding figure for males was 135.4 (95% CI 116.8 to 152.2).
Age-standardised incidence rate of normal mucosa per 100 000 person-years in Sweden 1990–2015. GAM, generalised additive model.
The ratio of the age-standardised incidence of CD to the incidence of duodenal/jejunal normal mucosa decreased in females from 0.189 (95% CI 0.156 to 0.229) in 1990 to 0.082 (95% CI 0.071 to 0.096) in 2015, and in males from 0.181 (95% CI 0.151 to 0.216) in 1990 to 0.071 (95% CI 0.062 to 0.083) in 2015 (figure 3).
Incidence ratio of coeliac disease to normal mucosa. GAM, generalised additive model.
Age
The mean age at CD diagnosis was 32 years (SD 25) for the entire study period. Examining the periods 1990–2000, 2001–2008 and 2009–2015 separately, the mean age was 32 years for each period. Figure 4 displays age-specific incidence rates of CD by 5-year age groups, with the highest rates seen in the youngest (0 to <5 years) children (females 72.0/100 000 (95% CI 70.0 to 74.1), males 39.7/100 000 (95% CI 38.3 to 41.2)). These rates continued to decline until the age of 20, after which rates in women continued to decline, but increased slightly in men to age 80, after which both sexes declined. From the age of 60, age-specific incidence rates were broadly similar between sexes. The number of patients with CD, stratified by sex, per age group are presented in the appendix (online supplemental tables S2 and S3).
Supplemental material
Age-specific incidence rate of coeliac disease in Sweden 1990–2015. GAM, generalised additive model.
Age-specific incidence rates were highest in the first age category (0 to <5 years). Online supplemental eFigures 4 and 5 show age-specific incidence rates for all age groups, stratified by sex, for both CD and normal mucosa, across the study period. Interestingly, the two-wave pattern seen in the overall age-standardised incidence rates (figure 1) was observed only in the youngest age category (0 to <5 years).
Supplemental material
Supplemental material
Examining rates for children 0 to <2 and 2 to <5 years separately, we found higher rates in the younger population (0 to <2 years), with 104.6/100 000 (95% CI 100.8 to 108.5) person-years in girls and 57.9/100 000 (95% CI 55.2 to 60.8) person-years in boys. These figures can be compared with 50.6/100 000 (95% CI 48.4 to 52.8) person-years in girls and 27.7/100 000 (95% CI 26.2 to 29.3) person-years in boys in the age group 2 to <5 years. Corresponding figures for normal mucosa were: 80.0/100 000 (95% CI 76.7 to 83.4) person-years in girls and 84.8/100 000 (95% CI 81.5 to 88.2) person-years in boys aged 0 to <2 years and 44.7/100 000 (95% CI 42.7 to 46.8) person-years in girls and 58.5/100 000 (95% CI 56.3 to 60.8) person-years in boys aged 2 to <5 years.
The mean age at first biopsy with normal mucosa was 44 years (SD 20). The age at first biopsy was similar throughout the study period. Figure 5 shows age-specific incidence rates of normal mucosa by 5-year age groups and that females had more biopsies than males. The incidence rate of normal mucosa peaked in women about age 20 (300–350/100 000 person-years) and then gradually decreased until age 80 (160/100 000 person-years), after which there was a dramatic decline. Men’s incidence rates increased rapidly but to a lower level (approximately 160/100 000 person-years). They then continued to increase more slowly to the age of 80, after which there was a decrease similar to that in women. The number of patients, stratified by sex, with normal mucosa per age group are presented in the appendix (online supplemental tables S4 and S5).
Age-specific incidence rate of normal mucosa in Sweden 1990–2015. GAM, generalised additive model.
The ratio of age-specific incidence of CD to the incidence of duodenal/jejunal normal mucosa was approximately 1.5 in females and 0.6 in males for infants. After that, the IRR decreased to 0.08 in females and 0.06 in males at about age 20 (online supplemental eFigure 3). The age-specific incidence ratios then stayed at this level throughout life.
Supplemental material
Sex
The incidence of CD was higher in females, who made up 63% of all cases, yielding a female/male age-adjusted IRR of 1.42 (95% CI 1.38 to 1.45). Figure 4 depicts age-specific rates stratified by sex. We found compelling evidence that the IRR between sexes varied with age (p<0.001) and that the IRR between females and males decreased with age.
Similarly, we found a higher incidence for normal mucosa in females, with a female/male age-adjusted IRR of 1.54 (95% CI 1.53 to 1.55). The IRR varied significantly by age (p<0.001) with attenuation in the younger (<10 years) and older (>65 years) age groups.
When adjusting for calendar period, we observed a female/male IRR of 1.75 (95% CI 1.70 to 1.79) in individuals with CD. We found convincing evidence (p<0.001) that the IRR between sexes varied by calendar period, with decreasing ratios at the beginning and end of the study period.
In the group having a normal mucosa, adjustment for calendar period yielded a female/male IRR of 1.58 (95% CI 1.56 to 1.59). We found evidence (p<0.001) that the IRR between sexes varied by calendar period, with the gap between sexes growing continuously throughout the study period, except for the last year.
Cumulative incidence
The lifetime risk of developing CD was 1.8% (95% CI 1.8% to 1.8%). Stratified by sex, the lifetime risk was 2.3% in females (95% CI 2.3% to 2.3%) and 1.4% in males (95% CI 1.4% to 1.4%).
Discussion
This nationwide population-based cohort study demonstrated an age-standardised incidence rate of 19.0/100 000 person-years from 1990 to 2015. Incidence rose appreciably in the early 1990s, reaching a peak for both sexes in 1994. After an initial decline, even higher incidence rates were seen in 2002–2003 in females and in 2006 in males. Finally, during the last decade of the study period, the mean annual age-standardised incidence rate was 20.8 per 100 000 person-years. These changes over time may be due to a combination of environmental factors and diagnostic trends. The downward trend observed in the last 3 years of the study period may in part reflect the implementation of new biopsy sparing diagnostic criteria (ESPGHAN, introduced to children in 2012 in Sweden27). In contrast, the prevalence of normal biopsy increased during the study period, except for the somewhat lower incidence rates in 2015. This finding suggests that the temporal pattern of CD was moderately independent of the level of CD investigation, as reflected by normal mucosa incidence.
As expected, we found higher incidence rates of CD in females than in males. However, it is striking that the graphs depicting the age-specific incidence rates (figure 4) in males and females follow each other, suggesting that similar factors are in force in both sexes.
Comparison with previous literature
One Swedish study demonstrated a stabilised CD incidence in Swedish children since the early 2000s,28 but that study was limited to children and ended follow-up in 2009. Our study’s plateauing incidence is supported by similar findings from the past 15 years in Finland in an adult population.12 Trends in Italy, the USA (children) and the UK also showed signs of stabilising diagnosis rates.29–31 One potential explanation for these patterns is that clinically identifiable CD approaches the actual occurrence of CD in some areas with high disease prevalence. However, this pattern is not universal, as even certain regions within the UK show a constant increase despite high incidence. For example, data from Southeast Scotland have shown a 13.0% increase in paediatric CD incidence per year since 1990, with the most recent estimate in 2016 at 36.0 per 100 000 person-years.19 32
Of additional interest is the contrast with Denmark, a geographically and demographically similar nation to other Scandinavian countries. When considering historical patterns and even more recent estimates (eg, 15.2 per 100 000 person-years in 2015–2016),33 CD incidence in Denmark has been comparatively lower than in Sweden. Regardless, nationwide rates have been consistently increasing from 1980 to 2016 by about 8.0% per year. Estonia, another country close in proximity, has seen comparatively low incidence rates in the paediatric population (eg, 3.1 per 100 000 person-years in 2006–2010). However, these rates have also been increasing at approximately 8.0% per year since 1976.18 Therefore, it is important to continue following trends in areas with high but stabilising and low but increasing incidence rates. Such an endeavour may uncover additional clues in disease aetiology and help assess targeted healthcare efforts to improve CD detection. Although separated in time, the increases are similar in degree, suggesting the gradual spread of some environmental factor(s) from one geographic area to another. Alternatively, the rate of normal mucosa was not reported in these studies, underscoring the extent to which underdiagnosis of CD remains unknown (and therefore may partly explain the comparatively lower estimates).
Interestingly, we found two incidence peaks during the study period, the first occurring in 1994 and the second in 2002–2003 in females and 2006 in males. A similar pattern was observed among Swedish children aged 0–1.9 years in a 2014 study28 suggesting a varying influence of unknown risk factors over time. This finding is corroborated by our results (online supplemental eFigure 4) where the youngest children, 0 to <5 years, was the only age group where the two-wave pattern seen in the overall age-standardised incidence rates was clearly observed. A 2009 Swedish paper found that patients born during the Swedish CD epidemic of the early 1990s had an increased risk to develop CD later in life.34 The authors of the latter study34 concluded this could be related to the practice of introducing gluten without concomitant breast feeding which was the recommendation at the time. It is possible that the second CD incidence peak (early-mid 2000s) might be due to the development and use of tissue transglutaminase antibodies,35 later followed by a decline due to a saturation in diagnosis of patients with a high likelihood of CD. However, of note, the following decrease in CD incidence was not paralleled by a decrease in normal mucosa incidence.
Our study shows that CD is not just a disease of the young. Already in 2009,22 we presented data showing that the median age at first diagnosis of CD (defined by first small intestinal biopsy with VA) was 30 years, with 6/10 patients diagnosed beyond childhood. Still, the current study revealed large differences in CD incidence by age. This finding is not surprising given that research has indicated that changes in infant feeding recommendations affect the risk of CD primarily in childhood.36 In 1985–1987, the CD incidence in children <2 years of age increased dramatically in Sweden to 200–240/100 000 person-years and then declined in 1995 to 50–60/100 000 person-years. No comparable change was seen in children aged ≥2 years. In contrast to the Ivarsson et al study,36 which was based on a subset of the Swedish child population and included retrospective data from 1973 to 1990 from some paediatric departments, our data were nationwide and prospectively recorded. Still, our data were limited to computerised data in which some pathology departments were fully computerised only in the mid-late 1990s. All these factors may have contributed to differences between studies.
Other factors may also have affected the incidence of CD. For instance, antibiotic use has been linked to later CD.37 In January 2014, the Stockholm County Council introduced rotavirus vaccination in infants, a practice later followed by other Swedish regions. While earlier data on the role of rotavirus and gastroenteritis for CD development is inconsistent,38 39 it was beyond the current study’s scope to explore the impact of rotavirus vaccination on CD incidence. During the study period, smoking fell sharply in Sweden. Between the late 1980s and 2016, the proportion of adults in Sweden who reported daily smoking decreased from 27% to 11%.40 Some data suggest that smoking protects against CD41 (and cessation could thereby drive an increase in incidence), but data from Sweden show no clear inverse association.42 43 It is outside the scope of this study to link changes in incidence to specific risk factors, but it may produce testable hypotheses.
Strengths and limitations
We used nationwide data from Sweden’s 28 pathology departments to calculate age-specific incidence rates for CD over 25 years. It is crucial for representative data that incidence data do not originate from tertiary centres or areas with special interest in CD (because diagnosis is not always evident and the condition may go undiagnosed for many years). A second strength is our comparison with normal mucosa. To our knowledge, this comparison has not been previously made and it puts CD incidence in context. This perspective is important when studying CD, given that individuals can remain undiagnosed, sometimes for their entire life. The initial increase of CD may largely be due to increased awareness and investigation and CD screening of high-risk groups has increased in the past decades.44
Still, other factors are likely to have played a role. For instance, screening for CD over time has revealed a real increase in several countries.45 46 Also, the incidence plateau just after 2002, despite increasing investigation of CD (as reflected in the increased biopsy rates showing normal mucosa), suggests a real change in incidence.
We had a follow-up of 25 years, allowing us to discern several temporal shifts in CD not likely to be due to administrative and diagnostic changes.
We defined CD as having small intestinal VA (Marsh III). This definition has high specificity. In a patient chart review 108/114 individuals with VA had CD, yielding a PPV of 95%.23 The high PPV was confirmed in a review of free text biopsy reports from 2009 to 2017.3 Of individuals with available data on CD serology, 88% had positive serologies at the time of diagnosis, similar to the 89% (n=166/187 tested) in the incidence study in Olmsted County.14
Our study has a high sensitivity for diagnosed CD, at least until 2012. In our validation study, 172/180 (96%) interviewed Swedish gastroenterologists and 68/68 (100%) paediatricians performed a small intestinal biopsy in at least 9/10 individuals before the diagnosis of CD.23 Following recommendations from ESPGHAN,27 the Swedish Society of Pediatrics adopted an option to diagnose CD without biopsy in selected children with a diagnosis in 2012. Considering the incidence of CD and normal mucosa in children <5 years, which both declined from 2012 (online supplemental eFigures 4 and 5), it seems that the non-biopsy option has gained popularity in recent years among Swedish paediatricians. This conclusion is corroborated by a 2019 questionnaire study that found that 86% of the participating clinics avoided endoscopy in children presenting with symptoms typical for CD and highly positive serology.47 We therefore urge caution when interpreting the incidence data of children beyond 2012. Also, this may, in part, explain the decreasing overall incidence of CD observed 2013–2015.
In the past decade, small intestinal inflammation without VA (Marsh I–II) in individuals with positive CD serology has also been accepted as evidence of CD by some physicians and researchers. We decided not to include inflammation in our definition of CD as this would distort the incidence patterns over time. Naturally, if patients with milder mucosal aberrations are diagnosed as having CD, this will increase the societal burden but may reduce the average burden of disease in patients (some of whom do not have VA). The risk of future complications in CD differs by underlying histopathology and, for instance, patients with inflammation have a lower risk of lymphoproliferative malignancy than those with VA.48 Had we used physician-assigned diagnoses of CD for our incidence study, an increase could have been expected just because of a change in disease criteria over time.
Our study did not include undiagnosed CD. Also, there is evidence to suggest that CD is a condition that remains largely undetected.49 Only universal testing can ascertain all such cases, and would lead to an even higher incidence than what we have shown. Except for limited regional investigations,50 there has been no large-scale screening study in Sweden that would have had impact on nationwide incidence rates. The findings from this study could indicate that in Sweden, the gap between diagnosed and undiagnosed CD has narrowed. Furthermore, the trends in incidence rates among other countries with high awareness and diagnostic capabilities will be important to gain further understanding of how these factors play a role in unmasking undiagnosed cases. Other limitations are that we did not have complete information on biopsies from birth to death in all individuals and neither did we have access to data on the total number of endoscopies which may, primarily, have affected the numbers of normal mucosa if the physician ruled out CD based on a visual inspection only. Neither did we have information on the indication for the biopsies.
To enable international comparisons with our data, we calculated age-standardised incidence rates based on the 2013 European age-standardised rates.
Finally, we cannot rule out the possibility that the decrease in 2015 in normal mucosa incidence is marginally artificial. Although we should have almost complete data in that year, the decrease did not start in 2012–2013 with the change in childhood diagnostic criteria but only in 2015. The decrease in 2015 may be due to insufficient reporting. Patterns in normal mucosa compared with the incidence of CD may also be influenced by the age distribution of these two groups: individuals in our study diagnosed with CD were relatively younger than those found to have normal mucosa. In recent years, older individuals have higher biopsy rates, but due to a lower probability of CD diagnosis compared with children, the incidence of CD appears to be stabilising. However, we cannot rule out some biopsy avoidance in the elderly, which will drive down the incidence rates in this population.
In conclusion, we found two waves of CD incidence in Sweden during the past two-and-a-half decades. The incidence increased until 2002–2003 for females and until 2006 for males, with an earlier peak around 1994. Since then, the overall incidence has slightly decreased, despite an increasing number of duodenal/jejunal biopsies, suggesting that increased awareness and histologic study of CD are unlikely to increase the incidence of CD in Sweden at this stage. The age-specific incidence rates by calendar period suggest that the observed two-wave pattern of CD incidence is driven by children aged 0 to <5 in that the first wave was clearly observed only in this age group. Across a lifetime, 1 in 44 females and 1 in 72 males in Sweden are expected to be diagnosed with CD.
Data availability statement
In accordance with Swedish regulation the data from this study are not publicly available.
Ethics statements
Patient consent for publication
Ethics approval
The study was approved by the Regional Ethics Committee, Stockholm, Sweden (Protocol no 2014/1287-31/4).
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @jamesa_king, @gilkaplan
Contributors DB contributed to analysis and interpretation of the data; drafting of the manuscript; statistical analysis. MSC contributed to analysis and interpretation of the data; statistical analysis. BR contributed to analysis and interpretation of the data; statistical analysis. All authors contributed to critical revision of the manuscript for important intellectual content. JFL contributed to study supervision; drafting of the manuscript; obtained funding; study concept and design; acquisition of the data.
Funding This work was supported by the Swedish Research Council (Ludvigsson; Grant number: 2020-01706) and by The Swedish Society of Medicine (Bergman, award number SLS-920581).
Disclaimer None of the funding organisations have had any role in the design and conduct of the study; in the collection, management and analysis of the data; or in the preparation, review and approval of the manuscript. The content is solely the responsibility of the authors.
Competing interests JFL coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). This study has received funding from Janssen corporation. BL reports grants from Celiac Disease Foundation, personal fees from Takeda, personal fees from Anokion, outside the submitted work. GGK has received honoraria for speaking or consultancy from Abbvie, Janssen, Pfizer and Takeda. He has received research support from Ferring, Janssen, Abbvie, GlaxoSmith Kline, Merck and Shire. He has been a consultant for Gilead. He shares ownership of a patent: TREATMENT OF INFLAMMATORY DISORDERS, AUTOIMMUNE DISEASE, AND PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. 7 Sept. 2018. JAM received study grants from Nexpep/ImmusanT, National Institutes of Health, Immunogenix, Takeda Pharmaceutical, Allakos, Oberkotter and Cour; consultancy fees from Bionix, Lilly Research Laboratory, Johnson & Johnson, Dr Schar USA, UCB Biopharma, Celimmune, Intrexon Corporation, Chugai Pharma, Kanyos and Boehringer Ingelheim; holds patents licensed to Evelo Biosciences; and receives royalties from Torax Medical.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.